CE Mark Certification in Kenya to sell clinical gadgets in the European Union (EU), you should acquire or apply CE marking for your item. CE Marking demonstrates that your clinical gadget conforms to the appropriate EU guidelines and empowers the commercialization of your items in 32 European nations. As a legitimate clinical gadget producer, you are liable for keeping up administrative consistence and getting CE checking for your item, whether or not you reevaluate any or all parts of your assembling activity.
With workplaces all through Europe, Emergo's administrative specialists can assist you with getting Marking for your clinical gadget and begin selling your item in the EU.
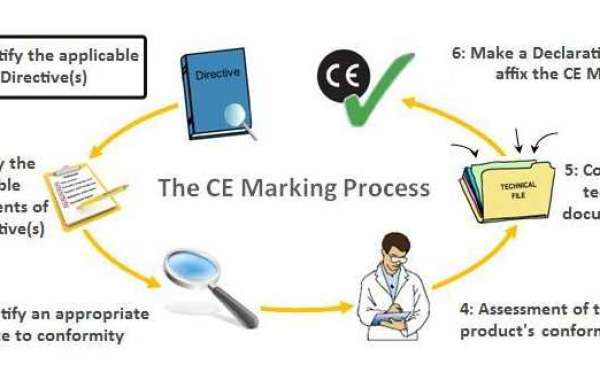
Step by step instructions to acquire European CE checking for your clinical gadget
CE is definitely not a quality imprint, yet consistence with EU Directives expects you to fulfill explicit guidelines of execution, quality, security, and viability for your item type. We have a definite graph clarifying the current European CE endorsement measure for clinical gadgets here. Nonetheless, the essential cycle follows these means:
- Figure out which EU Directive applies to your gadget: CE Mark Consultant in Sri Lanka Medical Devices Directive (93/42/EEC), In Vitro Diagnostic Devices Directive (98/79/EC), or Active Implantable Medical Devices Directive (90/385/EEC).
- Decide the grouping of your gadget. See our graph.
- Execute a Quality Management System, if relevant to your gadget. Most organizations use ISO 13485 to meet the necessities.
- Set up a CE Marking Technical File or a Design Dossier.
- Set up a Clinical Evaluation Report (CER) as per MEDDEV 2.7/1 rev4 and MDD (or MDR).
- Choose and delegate a European Authorized Representative to follow up for your sake inside the EU in the event that you have no actual area in Europe.
- Have your QMS and Technical File/Design Dossier reviewed by a Notified Body, except if your gadget is Class I, isn't sterile, and has no estimating capacity.
- Get CE Marking and ISO 13485 declarations from your Notified Body.
- Set up a Declaration of Conformity (DoC), which expresses that your gadget consents to the proper Directive.
Emergo can assist you with acquiring EU CE Marking for your clinical gadget
Emergo has a grounded presence CE Mark Services in Thailand in the EU with workplaces in the UK, Germany, France, and The Netherlands. We have helped many clinical gadget makers with CE consistence for Europe. Our administrations include:
- Help with item characterization
- Confirmation of pertinent norms and testing necessities
- Specialized File or Design Dossier gathering, or audit of your records
- Audit of existing showcasing materials, marking, and client manual data to guarantee consistence and consistency
- Check of consistence with Essential Requirements
- Planning of Clinical Evaluation Report dependent on gave clinical information
- Execution, adjustment, and upkeep of a quality framework (ordinarily ISO 13485) that will meet European and other worldwide prerequisites
- Approved Representative administrations in Europe
- Hazard evaluation and the executives (ISO 14971)
- Improvement of carefulness and post-market reconnaissance techniques
How to get CE Mark Consultants in UK?
We are providing Service for CE Mark Consultant Services in UK with extensive expertise and experience in all International Restriction of Hazardous Substances Standards. For Certification and Implementation of the Standards in your organization, reach Certvalue – CE Consultants contact us at +7760173623 or you can fill the form here, our experts will call you and guide for Successful Certification. Would be happy to assist your company in the CE Certification process to send your research after contact@certvalue.com.